Understanding the Bohr Model of the Atom
The Development of the Bohr Model
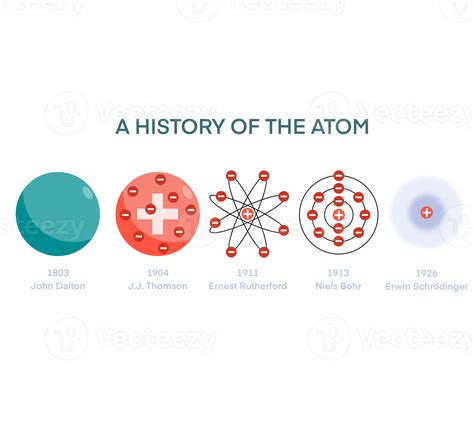
The Bohr model of the atom was developed by Danish physicist Niels Bohr in 1913. At the time, the most widely accepted model of the atom was the Rutherford model, which described the atom as a small, dense nucleus surrounded by a cloud of electrons. However, this model was unable to explain several key features of atomic behavior, including the emission and absorption spectra of atoms.
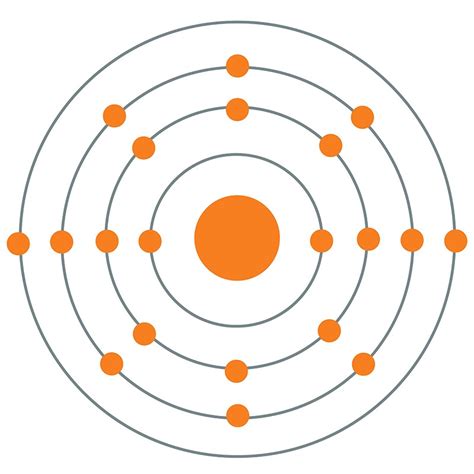
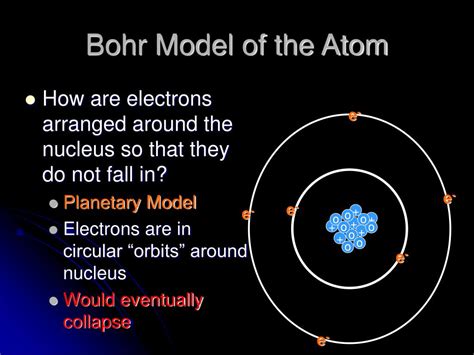
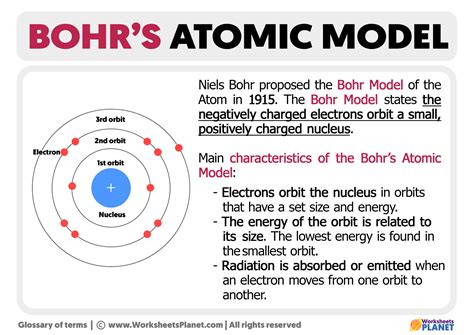
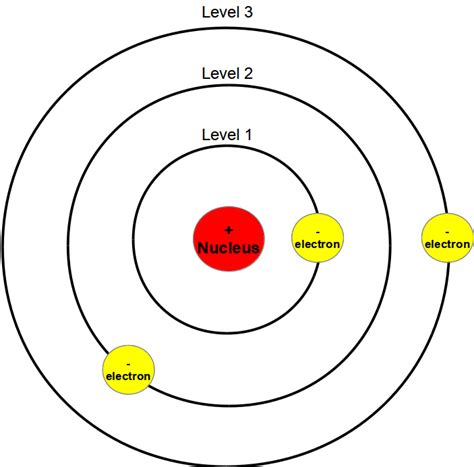
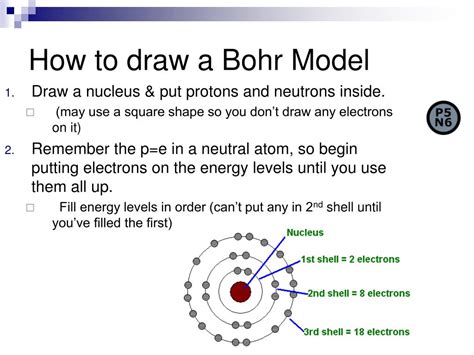
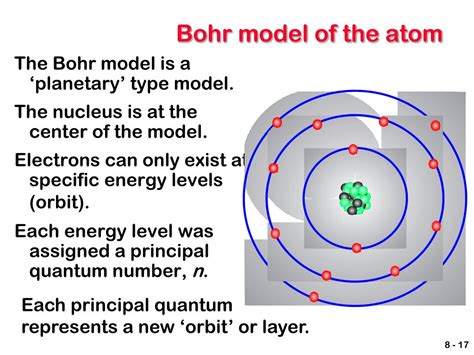
Bohr’s model was a significant improvement over the Rutherford model. It posited that electrons occupy specific energy levels, or shells, around the nucleus. Each energy level corresponds to a specific orbit, or distance, from the nucleus. Bohr’s model also introduced the concept of electron spin, which is a fundamental property of electrons that plays a crucial role in determining the behavior of atoms.
Key Components of the Bohr Model
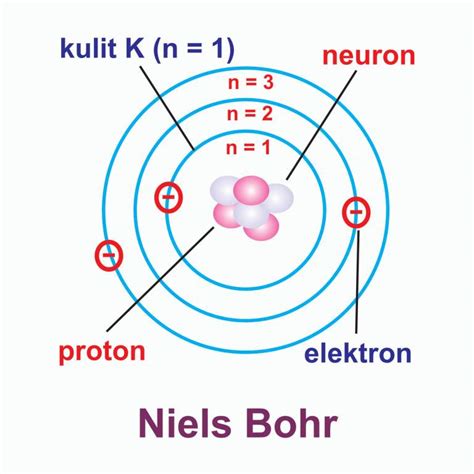
The Bohr model consists of several key components:
- Nucleus: The nucleus is the central part of the atom, containing most of its mass. It is composed of protons and neutrons.
- Electron energy levels: Electrons occupy specific energy levels, or shells, around the nucleus. Each energy level corresponds to a specific orbit, or distance, from the nucleus.
- Electron spin: Electron spin is a fundamental property of electrons that plays a crucial role in determining the behavior of atoms. Electrons can have a spin of +1⁄2 or -1⁄2.
- Quantum leaps: According to the Bohr model, electrons can only occupy specific energy levels. When an electron gains or loses energy, it can jump from one energy level to another. This is known as a quantum leap.
How the Bohr Model Works
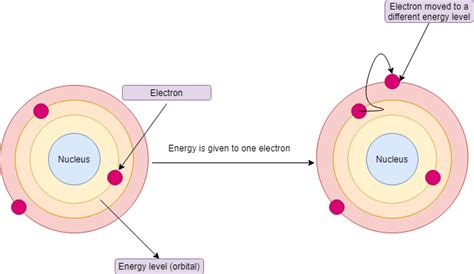
The Bohr model works as follows:
- Electrons occupy energy levels: Electrons occupy specific energy levels, or shells, around the nucleus. Each energy level corresponds to a specific orbit, or distance, from the nucleus.
- Electrons absorb and emit energy: When an electron gains energy, it can jump to a higher energy level. When an electron loses energy, it can jump to a lower energy level.
- Quantum leaps occur: When an electron gains or loses energy, it can jump from one energy level to another. This is known as a quantum leap.
- Electron spin affects energy levels: Electron spin affects the energy levels that electrons can occupy. Electrons with a spin of +1⁄2 can occupy different energy levels than electrons with a spin of -1⁄2.
💡 Note: The Bohr model is a simplified model of the atom. While it is still widely used today, it has been largely superseded by more advanced models, such as the quantum mechanical model.
Limitations of the Bohr Model
The Bohr model has several limitations:
- Oversimplification: The Bohr model is a simplified model of the atom. It does not take into account many of the complexities of atomic behavior.
- Limited accuracy: The Bohr model is not highly accurate. It is unable to explain many of the subtleties of atomic behavior.
- Inability to explain chemical bonding: The Bohr model is unable to explain chemical bonding, which is a fundamental aspect of chemistry.
Impact of the Bohr Model
Despite its limitations, the Bohr model has had a significant impact on our understanding of the atom:
- Revolutionized our understanding of the atom: The Bohr model revolutionized our understanding of the atom, introducing the concept of electron energy levels and electron spin.
- Led to the development of quantum mechanics: The Bohr model laid the foundation for the development of quantum mechanics, which is a more advanced and accurate model of the atom.
- Explained many of the features of atomic behavior: The Bohr model was able to explain many of the features of atomic behavior, including the emission and absorption spectra of atoms.
Comparison to Other Atomic Models
The Bohr model can be compared to other atomic models, such as the Rutherford model and the quantum mechanical model:
- Rutherford model: The Rutherford model is a simpler model of the atom that does not take into account electron energy levels or electron spin.
- Quantum mechanical model: The quantum mechanical model is a more advanced and accurate model of the atom that takes into account the complexities of atomic behavior.
| Model | Key Features | Limitations |
|---|---|---|
| Bohr Model | Electron energy levels, electron spin, quantum leaps | Oversimplification, limited accuracy, inability to explain chemical bonding |
| Rutherford Model | Small, dense nucleus, cloud of electrons | Inability to explain emission and absorption spectra, electron energy levels, and electron spin |
| Quantum Mechanical Model | Wave-particle duality, uncertainty principle, Schrödinger equation | Highly complex and difficult to understand, requires advanced mathematical knowledge |
The Bohr model of the atom is a significant improvement over earlier models, introducing the concept of electron energy levels and electron spin. While it has several limitations, it has had a profound impact on our understanding of the atom and has laid the foundation for the development of quantum mechanics.
In the end, the Bohr model is a fundamental concept in chemistry and physics that has helped us understand the behavior of atoms. While it is not a perfect model, it has paved the way for more advanced models and has revolutionized our understanding of the atomic world.
What is the Bohr model of the atom?
+
The Bohr model is a model of the atom that describes the atom as a small, dense nucleus surrounded by electrons in specific energy levels, or shells.
What are the key components of the Bohr model?
+
The key components of the Bohr model are the nucleus, electron energy levels, electron spin, and quantum leaps.
What are the limitations of the Bohr model?
+
The Bohr model has several limitations, including oversimplification, limited accuracy, and an inability to explain chemical bonding.



