Calcium Atom Structure with Bohr Model

Understanding the Calcium Atom Structure using the Bohr Model
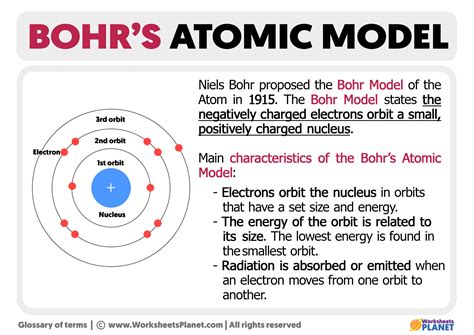
The Bohr model, developed by Niels Bohr in 1913, is a simplified model of an atom that describes the arrangement of electrons around the nucleus. In this article, we will explore the calcium atom structure using the Bohr model, and understand the distribution of electrons within the atom.
What is the Bohr Model?
The Bohr model is a planetary model of an atom, where the nucleus is at the center, and the electrons orbit around it in fixed energy levels or shells. The model assumes that electrons occupy specific energy levels, or shells, and that they jump from one energy level to another by emitting or absorbing energy.
Calcium Atom Structure
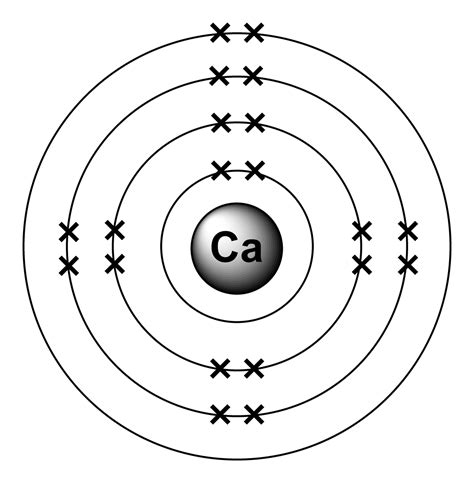
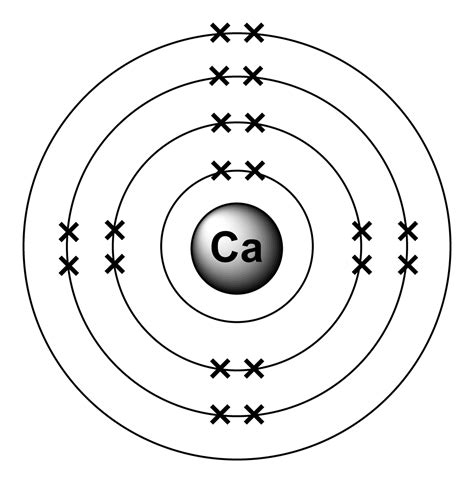
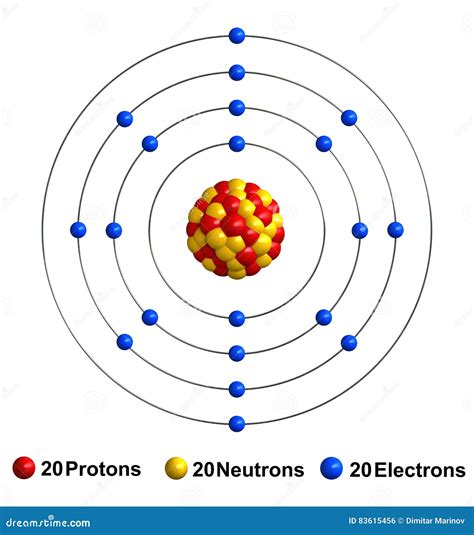
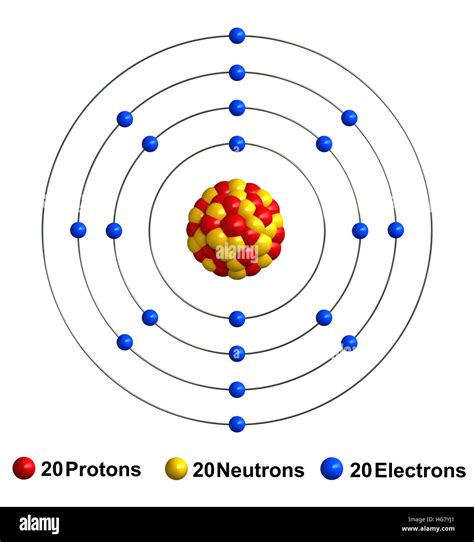
Calcium is an alkaline earth metal with an atomic number of 20 and an atomic mass of 40.08 u (unified atomic mass units). The calcium atom has 20 protons, 20 neutrons, and 20 electrons.
🔍 Note: The atomic number of an element is equal to the number of protons in its atomic nucleus, while the atomic mass is the sum of protons and neutrons.
Using the Bohr model, we can represent the calcium atom structure as follows:
- Nucleus: 20 protons and 20 neutrons
- Electron Shells: 4 energy levels or shells, with a total of 20 electrons
Electron Configuration of Calcium
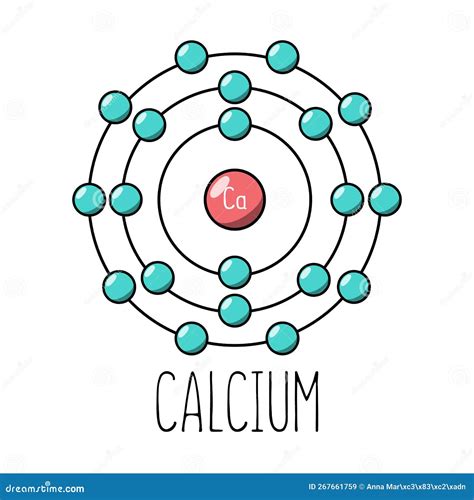
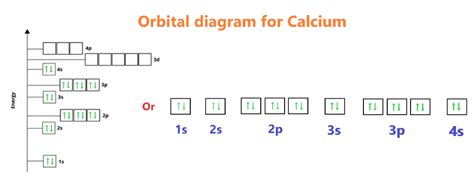
The electron configuration of an atom describes the distribution of electrons within the atom. The electron configuration of calcium is:
1s² 2s² 2p⁶ 3s² 3p⁶ 4s²
This configuration indicates that:
- The first energy level (1s) has 2 electrons
- The second energy level (2s) has 2 electrons
- The second energy level (2p) has 6 electrons
- The third energy level (3s) has 2 electrons
- The third energy level (3p) has 6 electrons
- The fourth energy level (4s) has 2 electrons
Calcium Atom Diagram
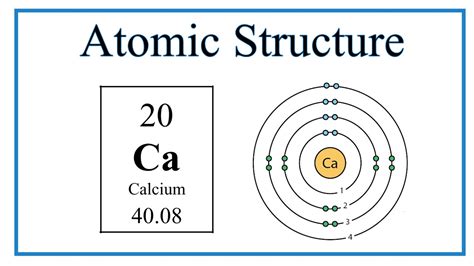
Here is a simplified diagram of the calcium atom structure using the Bohr model:
| Energy Level | Electrons |
|---|---|
| 1s | 2 |
| 2s | 2 |
| 2p | 6 |
| 3s | 2 |
| 3p | 6 |
| 4s | 2 |
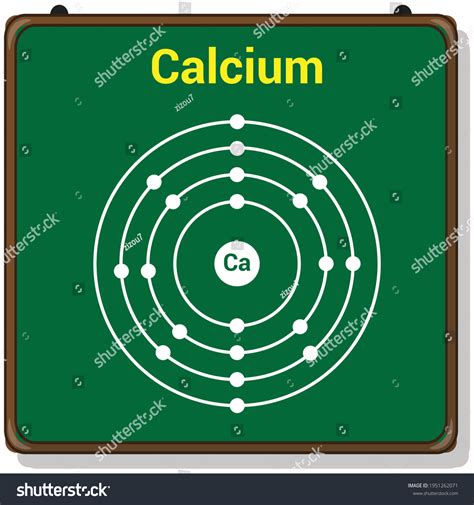
Conclusion
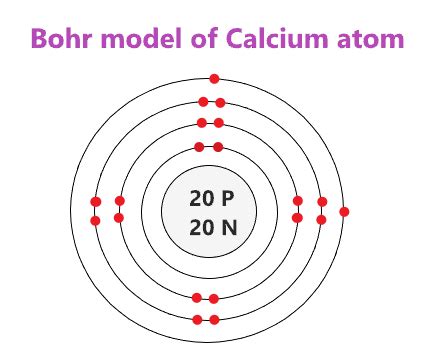
In this article, we have explored the calcium atom structure using the Bohr model. We have seen that the calcium atom has 20 protons, 20 neutrons, and 20 electrons, and that the electrons are distributed within four energy levels or shells. Understanding the electron configuration and structure of an atom is crucial in chemistry and physics, and the Bohr model provides a simplified and intuitive way to visualize the atomic structure.
What is the atomic number of calcium?
+
The atomic number of calcium is 20.
How many energy levels does the calcium atom have?
+
The calcium atom has 4 energy levels or shells.
What is the electron configuration of calcium?
+
The electron configuration of calcium is 1s² 2s² 2p⁶ 3s² 3p⁶ 4s².