5 Insights into Liquid Methanol Structure
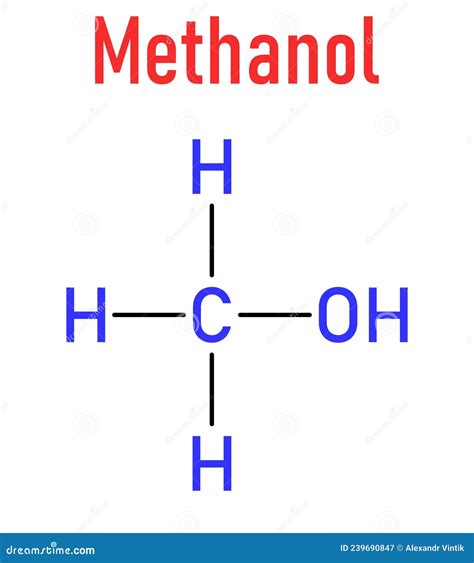
Understanding the Molecular Structure of Liquid Methanol
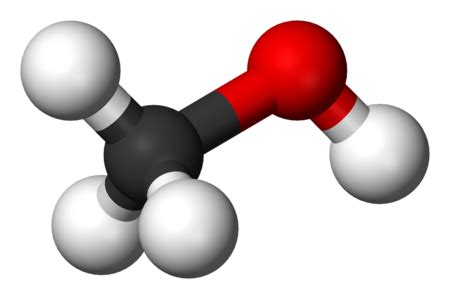
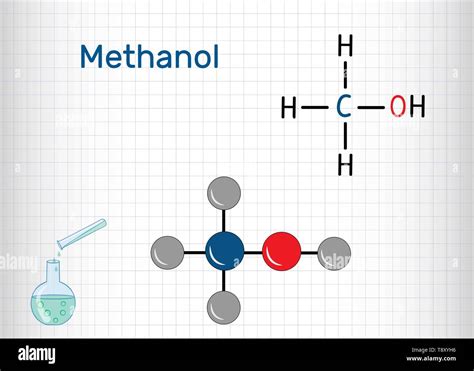
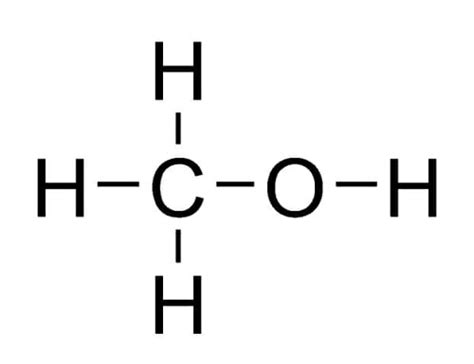
Methanol, also known as methyl alcohol, is a simple alcohol compound with the chemical formula CH3OH. It is a versatile chemical with a wide range of applications, including as a fuel, a solvent, and an antifreeze. In its liquid state, methanol exhibits a complex molecular structure that is influenced by intermolecular forces and hydrogen bonding. In this article, we will delve into the molecular structure of liquid methanol and explore five key insights that provide a deeper understanding of its properties and behavior.
Insight 1: Hydrogen Bonding and Intermolecular Forces

Liquid methanol is characterized by strong hydrogen bonding between its molecules. Hydrogen bonding is a type of intermolecular force that arises from the interaction between the hydrogen atom of one molecule and the oxygen atom of another molecule. In methanol, the hydrogen atom bonded to the oxygen atom is highly polar, creating a partial positive charge that is attracted to the partial negative charge on the oxygen atom of another molecule. This hydrogen bonding is responsible for the relatively high boiling point of methanol (64.7°C) compared to other alcohols of similar molecular weight.
🔍 Note: Hydrogen bonding plays a crucial role in the physical and chemical properties of methanol, including its viscosity, surface tension, and solubility.
Insight 2: Molecular Association and Clustering
Studies have shown that liquid methanol exhibits molecular association and clustering, where molecules form small aggregates or clusters due to hydrogen bonding. These clusters can range in size from a few molecules to several dozen molecules, depending on the temperature and concentration of the solution. The formation of clusters affects the physical properties of liquid methanol, such as its viscosity and density, and also influences its chemical reactivity.
- Cluster formation increases the viscosity of liquid methanol, making it more resistant to flow.
- Clustering also affects the solubility of methanol, as the formation of clusters can reduce the number of available solvent molecules.
Insight 3: Dipolar Nature of Methanol Molecules

Methanol molecules have a dipolar nature, meaning they have a permanent electric dipole moment due to the difference in electronegativity between the carbon and oxygen atoms. This dipolar nature allows methanol molecules to interact with other polar molecules, such as water, and participate in hydrogen bonding. The dipolar nature of methanol also contributes to its solubility in water and other polar solvents.
| Property | Value |
|---|---|
| Dipole moment | 1.69 D |
| Dielectric constant | 33.6 |
Insight 4: Temperature-Dependent Structure

The molecular structure of liquid methanol is highly dependent on temperature. At low temperatures, the molecules form a more ordered structure, with stronger hydrogen bonding and clustering. As the temperature increases, the molecules gain kinetic energy and begin to vibrate more, disrupting the hydrogen bonding and clustering. This temperature-dependent structure affects the physical properties of liquid methanol, such as its viscosity and density.
🔥 Note: The temperature-dependent structure of liquid methanol is important in understanding its behavior in various applications, such as fuel cells and biofuels.
Insight 5: Impact on Chemical Reactivity
The molecular structure of liquid methanol also influences its chemical reactivity. The hydrogen bonding and clustering in liquid methanol can affect the reaction rates and mechanisms of chemical reactions involving methanol. For example, the formation of clusters can reduce the number of available reactant molecules, affecting the reaction rate. Additionally, the dipolar nature of methanol molecules can influence the binding of reactants to catalysts, affecting the reaction mechanism.
- Hydrogen bonding and clustering can reduce the reaction rate by reducing the number of available reactant molecules.
- The dipolar nature of methanol molecules can influence the binding of reactants to catalysts, affecting the reaction mechanism.
In summary, the molecular structure of liquid methanol is complex and influenced by intermolecular forces, hydrogen bonding, and dipolar nature. Understanding these insights provides a deeper appreciation for the properties and behavior of liquid methanol, which is essential for its various applications.
What is the boiling point of methanol?
+
The boiling point of methanol is 64.7°C.
Why is hydrogen bonding important in liquid methanol?
+
Hydrogen bonding is important in liquid methanol because it affects its physical properties, such as viscosity and surface tension, and also influences its chemical reactivity.
What is the effect of temperature on the molecular structure of liquid methanol?
+
The molecular structure of liquid methanol is highly dependent on temperature. At low temperatures, the molecules form a more ordered structure, while at high temperatures, the molecules gain kinetic energy and begin to vibrate more, disrupting the hydrogen bonding and clustering.



