Why Was Flexeril Discontinued by the FDA

The FDA's Decision to Discontinue Flexeril: Understanding the Reasons

Flexeril, also known as cyclobenzaprine, is a muscle relaxant medication that was widely used to treat muscle spasms and pain. However, in recent years, the FDA has taken steps to limit the use of Flexeril, and it is no longer available in its original form. But why was Flexeril discontinued by the FDA? In this article, we will explore the reasons behind this decision and what it means for patients who rely on this medication.
History of Flexeril and Its Uses

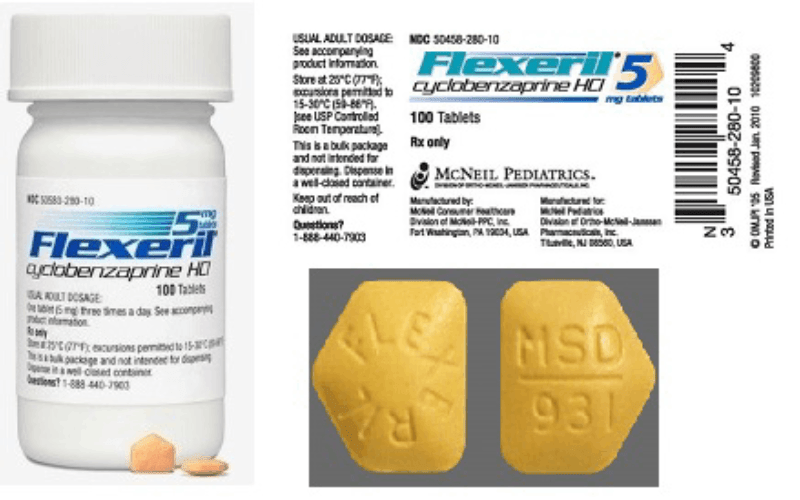
Flexeril was first approved by the FDA in 1977 as a treatment for muscle spasms and pain. It was marketed as a 5mg and 10mg tablet, and it quickly gained popularity among healthcare providers and patients alike. Flexeril was often prescribed to treat conditions such as back pain, fibromyalgia, and musculoskeletal injuries.
Concerns Over Flexeril's Safety and Efficacy

Over the years, concerns have been raised about the safety and efficacy of Flexeril. Some studies have suggested that the medication may not be as effective as other muscle relaxants, and that it may have a higher risk of side effects. Additionally, there have been reports of patients becoming dependent on Flexeril and experiencing withdrawal symptoms when they try to stop taking it.
FDA's Decision to Limit Flexeril's Use
In 2019, the FDA announced that it would be limiting the use of Flexeril due to concerns over its safety and efficacy. The agency cited studies that showed the medication was not effective in treating muscle spasms and pain, and that it had a higher risk of side effects, including sedation, dizziness, and dry mouth.
As a result, the FDA decided to approve a new formulation of Flexeril, called Amrix, which is a 15mg and 30mg extended-release capsule. Amrix is designed to be taken once daily, and it is intended to provide longer-lasting relief from muscle spasms and pain.
🚨 Note: The FDA's decision to limit Flexeril's use does not mean that the medication is no longer available. However, it does mean that healthcare providers will need to be more cautious when prescribing it, and that patients may need to try alternative treatments.
Alternative Treatments for Muscle Spasms and Pain

If you are currently taking Flexeril and are concerned about the FDA’s decision, there are alternative treatments that you can discuss with your healthcare provider. Some options include:
- Other muscle relaxants, such as baclofen or tizanidine
- Over-the-counter pain relievers, such as acetaminophen or ibuprofen
- Physical therapy or massage to help relieve muscle tension
- Alternative therapies, such as acupuncture or chiropractic care
What Does the Future Hold for Flexeril?

The future of Flexeril is uncertain, and it remains to be seen whether the medication will continue to be available in some form. However, one thing is clear: the FDA is committed to ensuring that medications are safe and effective, and that patients have access to the best possible treatments.
As research continues to evolve, we may see new formulations or uses for Flexeril emerge. In the meantime, patients who rely on this medication should talk to their healthcare provider about alternative treatments and what the FDA’s decision means for their care.
What is Flexeril, and what was it used for?
+
Flexeril, also known as cyclobenzaprine, is a muscle relaxant medication that was widely used to treat muscle spasms and pain.
Why did the FDA limit Flexeril's use?

+
The FDA limited Flexeril's use due to concerns over its safety and efficacy. Studies showed that the medication may not be as effective as other muscle relaxants, and that it may have a higher risk of side effects.
What are some alternative treatments for muscle spasms and pain?

+
Alternative treatments for muscle spasms and pain include other muscle relaxants, over-the-counter pain relievers, physical therapy or massage, and alternative therapies like acupuncture or chiropractic care.
In summary, the FDA’s decision to limit Flexeril’s use is a response to concerns over its safety and efficacy. While the medication is no longer available in its original form, patients can discuss alternative treatments with their healthcare provider. As research continues to evolve, we may see new formulations or uses for Flexeril emerge.